
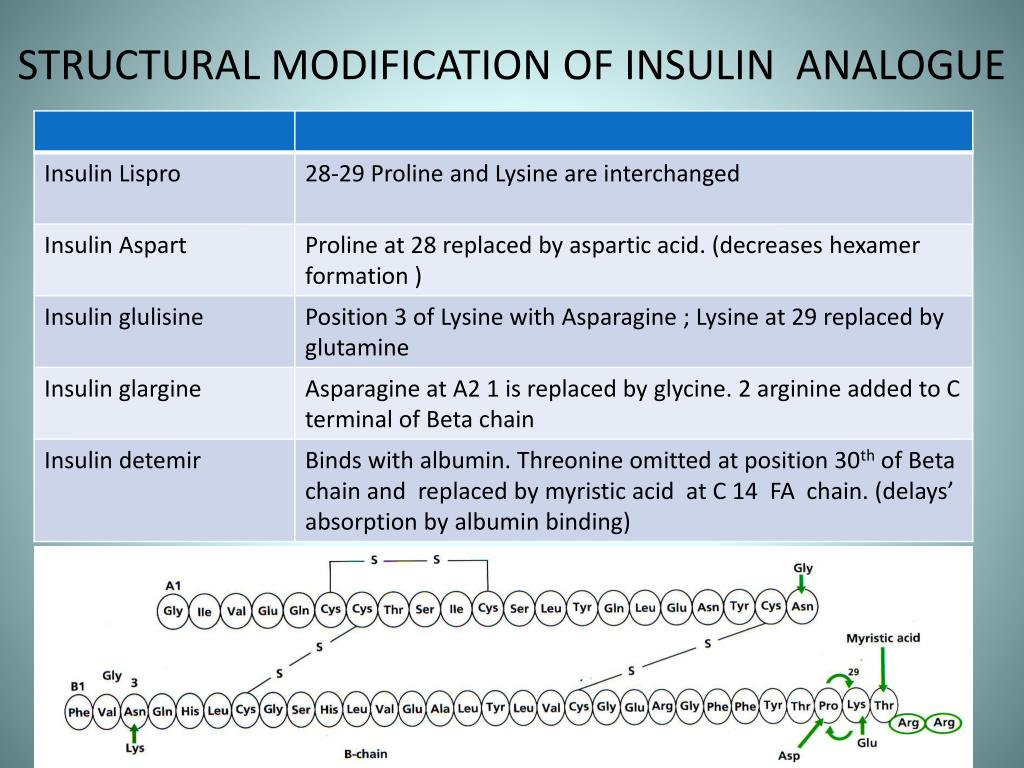

Human insulins are typically compared against analog insulins. Learn more about the past, present and future of insulin here. There are also some premixed combinations available. Analog insulins can be long and fast-acting. ” They are synthetic insulins that act like human insulin. in 1996 was Lispro, followed by Aspart in 2000 and Glulisine in 2004.Īnalog insulins are designed to “ mimic the body’s natural pattern of insulin release. The first short-acting insulin that was approved for use in the U.S. Since they have a different chemical structure than human insulin, they lower your blood sugar more quickly once injected. Analog insulins are easily absorbed from fatty tissue, making them more predictable. These examples all fall into categories of insulin called Aspart, Glulisine and Lispro. They have had a greater impact on people with diabetes since the early 2000s, replacing prescription requests for many human insulins. Analog insulins are created in labs by growing insulin proteins within E-coli bacteria (Escherichia coli) -similar to how human insulins are made. Analog insulins are different from biosimilars and are used as reference products. However, the interest in biosimilars continues to grow throughout the world! What are analog insulins?Īnalog insulins take up a lot more space in the healthcare market than biosimilars. As of February 2022, only 34 biosimilar products are approved in the U.S. Since biosimilar products come from natural sources and are not exact copies, they must be thoroughly investigated and studied before they get into the hands of patients. Getting biosimilars approved is not easy because of the FDA’s rigorous standards. Few biosimilars are on the market today because of patent litigation settlements. There is not as much information, research, awareness or availability compared to analog insulins. This is another long-acting insulin that is similar to Lantus insulin.ĭespite biosimilars being attractive to patients and physicians for their safety and cost-effectiveness, there are not many biosimilars approved for use in the U.S. The second FDA-approved “Insulin glargine and insulin glargine-yfgn are biologic medications (medications made from living organisms).” () Examples include: Toujeo SoloStar, Lantus Solostar and Basaglar KwikPen.insulin glargine biosimilar, Rezvoglar, was approved in December 2021. The FDA approved the first biosimilar insulin product, Semglee, in July 2021 for people with diabetes. Why aren’t many biosimilar insulins available?īiosimilar medications were first approved under section 351(K) of the Public Health Service Act for biosimilar products “ shown to be biosimilar or interchangeable with an FDA-licensed reference product. Biosimilar insulins are just as safe and effective for people with diabetes to use. Reference products are compared against any proposed biosimilars that enter the healthcare market. Some states have laws where biosimilar insulin can only be prescribed if it is lower in cost than its brand-name equivalent.īiosimilars have “ no clinically meaningful difference ” from the existing FDA-approved original product, also known as a “reference product.” A reference product is a single biological product that has already been approved by the FDA. Because pharmacy laws and regulations vary from state to state, so do interchangeability standards. However, not all states offer interchangeable medication options. (This works in the same way as your pharmacist substituting name-brand test strips for generic ones, for example.) You shouldn’t need to call your prescriber in order to switch your insulin prescription with a biosimilar at the pharmacy counter. These are intended to be easily interchanged with Lantus, a long-acting insulin by Sanofi.īiosimilars are supposed to be interchangeable with frequently-prescribed insulin products to reduce healthcare costs. Original insulins are “ FDA-approved, branded biologic used as a reference product during approval.” Biologic products are “ generally large, complex molecules that are often produced through biotechnology in a living system, such as a microorganism, plant cell or animal cell.” What are biosimilar insulins?Ĭurrent biosimilar insulins are biologically similar to other long-acting insulins on the market. In this guide, we break down the differences between biosimilar, human and analog insulins. In today’s insulin market, there are categories that every type of insulin falls under. If you or a loved one are struggling to afford or access insulin, click here. Editor’s Note: People who take insulin require consistently affordable and predictable sources of insulin at all times.


 0 kommentar(er)
0 kommentar(er)
